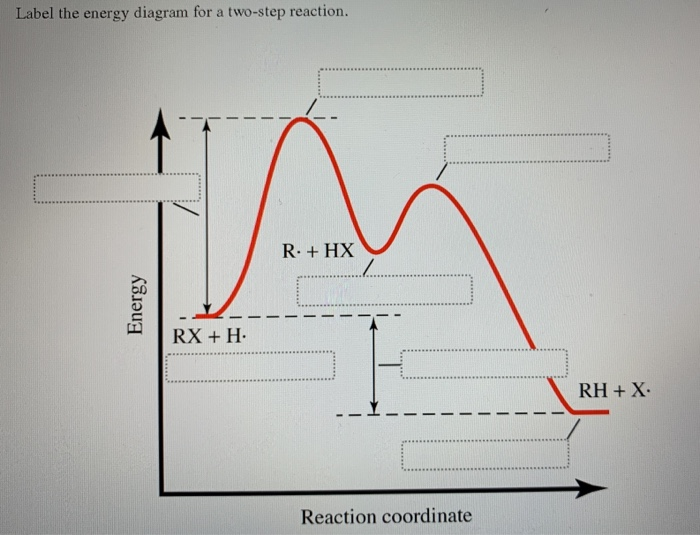
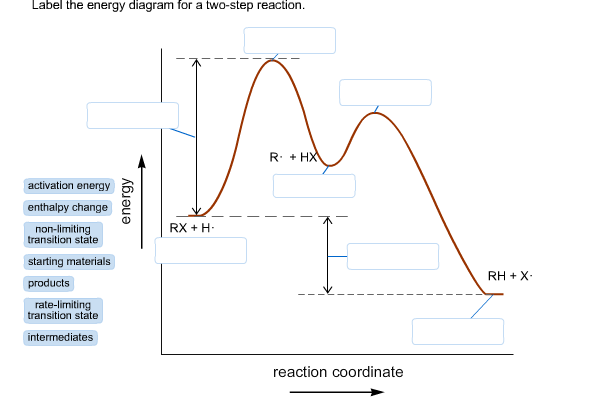
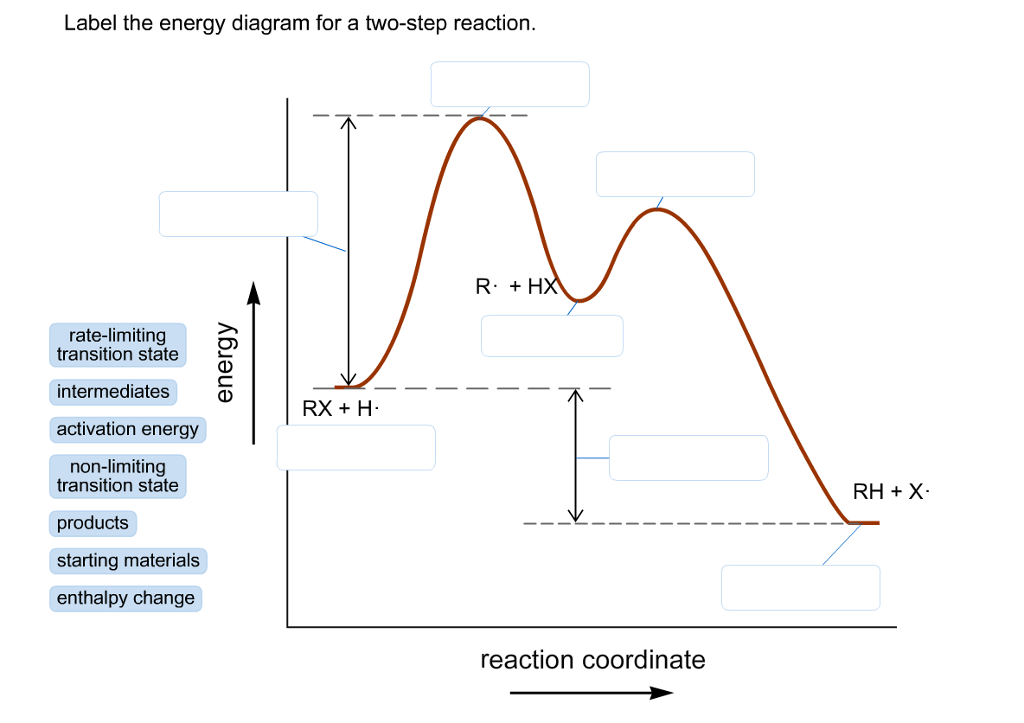
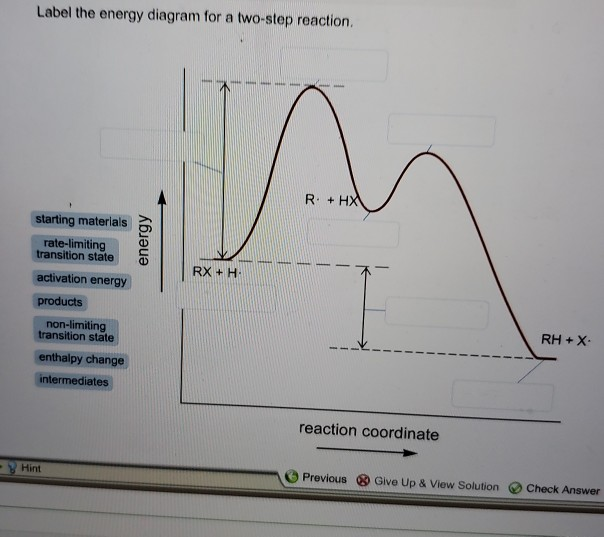
42 label the energy diagram for a two-step reaction.
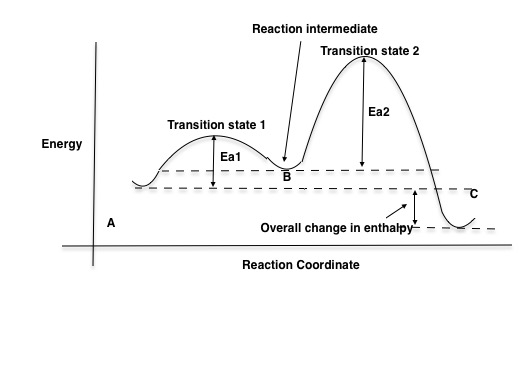
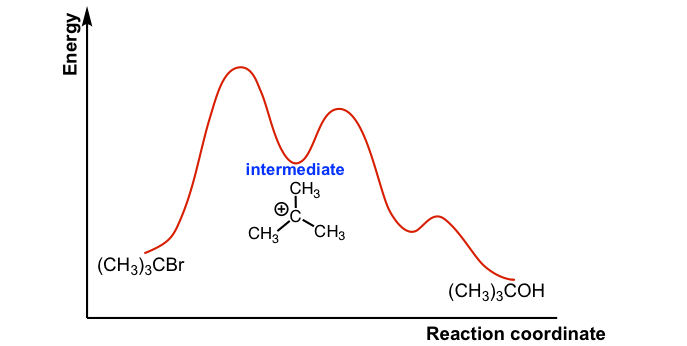
Answered: Draw a reaction energy diagram for a… | bartleby Science Chemistry Q&A Library Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate, transition states, ΔH, and activation energies. Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall.
Draw an energy diagram for a two-step reaction, $$ A \righ | Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw an energy diagram for a two-step reaction, $$ A \rightarrow B \rightarrow C, $$ where the relative energy of these compounds is C < A < B, and the conversion of $$ B \rightarrow C $$ is rate-determining..
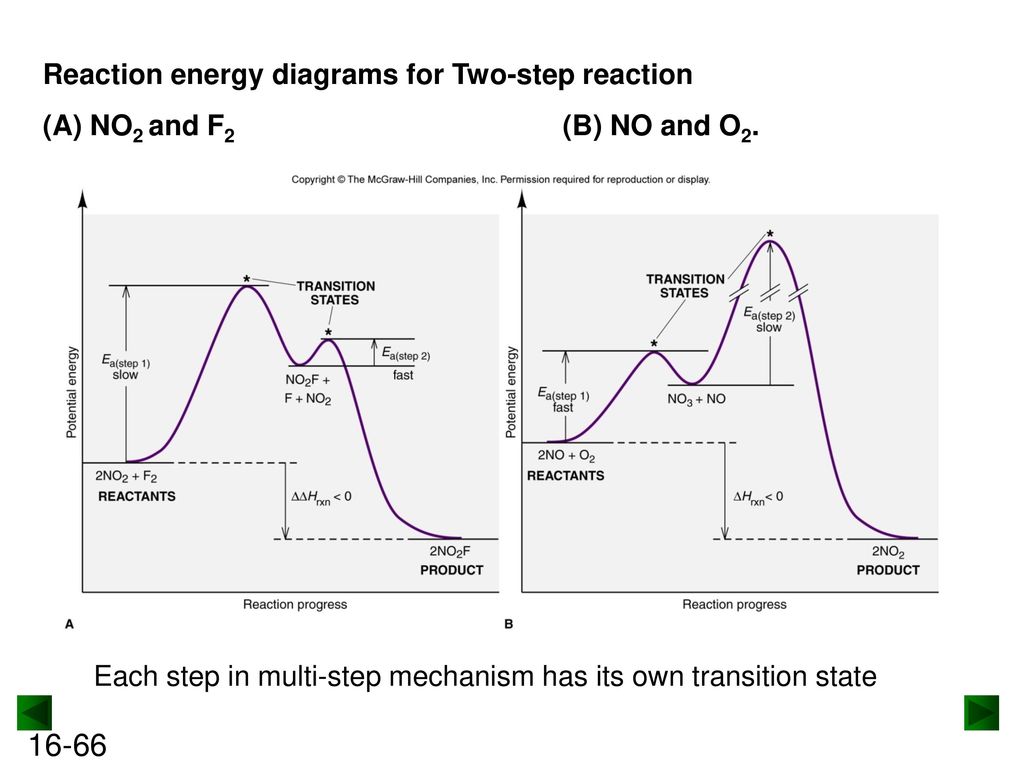
Label the energy diagram for a two-step reaction.
Label the energy diagram for a two-step reaction. Draw a labeled reaction-energy diagram for a two-step reaction with an early first transition state and a late second transition state. State whether the transition states resemble the starting material, intermediate, or product. Repeat the exercise for a; Draw a stepwise mechanism for the reaction depicted below. Mechanisms and Potential Energy Diagrams | Chemistry for Non-Majors ... A potential energy diagram for a two-step reaction is shown and labeled. Practice View the section on two-step reactions at the site below and then do the self-test (both buttons are at the top of the slide). Don't worry about - just consider it an indication of activation energy as is in the diagram above. Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
Label the energy diagram for a two-step reaction.. Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1 Answered: Sketch a qualitative reaction energy… | bartleby Sketch a qualitative reaction energy diagram for a chemical reaction with and without a catalyst. Assume the uncatalyzed reaction is endothermic. Note: Because the sketches are only qualitative, the energies in them don't have to be exact. They only have to have the right relationship to each other. For example, if one energy is less than ... Energy Diagrams of Reactions | Fiveable The energy diagrams below show what should be known for the test. Before looking at the specifics of each, you should be aware of a few terms: Activation Energy - Energy necessary for the reaction to occur. Activated Complex - The maximum point of energy, where the reactants turn into the products and the reaction occurs. Solved: Sketch an energy diagram for a two-step reaction in which ... Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram corresponding to reactant, product, intermediate, overall Δ G‡, and overall Δ G °. Step-by-step solution 91% (11 ratings) for this solution Step 1 of 4
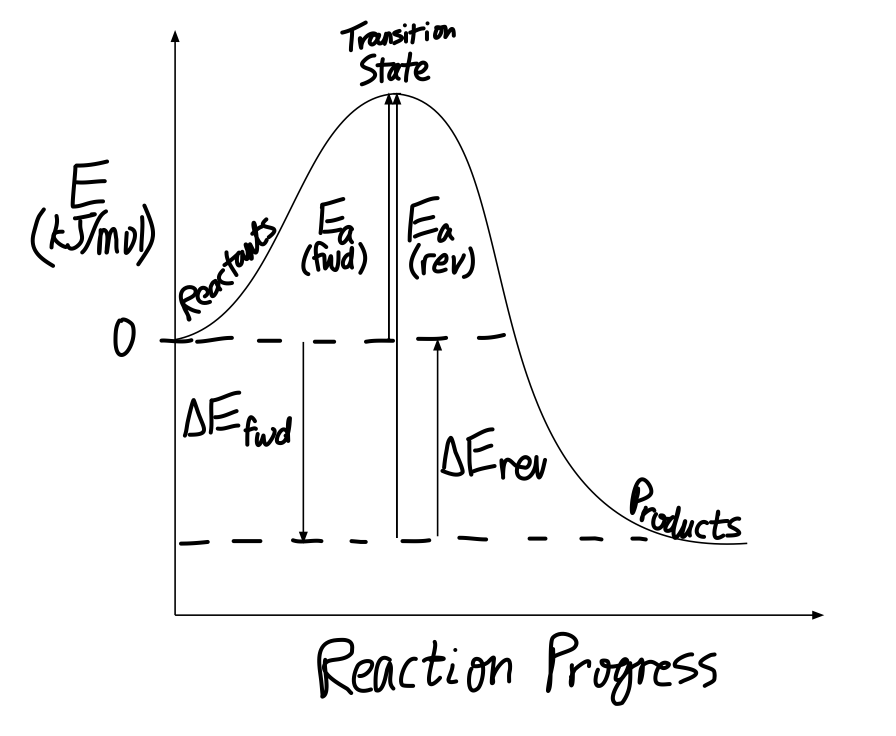
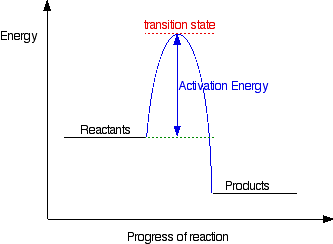
Energy Profiles (Energy Diagrams) Chemistry Tutorial - AUS-e-TUTE ⚛ An energy profile is a diagram representing the energy changes that take place during a chemical reaction. ⚛ Enthalpy change, ΔH, is the amount of energy absorbed or released by a chemical reaction. (2) On an energy profile, the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products ... How can I draw activation energy in a diagram? | Socratic 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4. Draw and label the activation energy. Draw a horizontal line from the highest part of the curve towards the vertical axis. Labeling an Energy Diagram Diagram | Quizlet Starting ingredients for Forward reaction. Forward Activation Energy (Ea) Energy required to break the bonds between atoms for the FORWARD reaction. Enthalpy (∆H) Heat of reaction; PE products - PE reactants. Activation Complex. Point of reaction in which all bonds between atoms are broken and atoms are free to recombine. Answered: Consider the following energy diagram… | bartleby How many steps are present in the reaction mechanism? d. Label each step of the mechanism as endothermic or exothermic. e. Label the overall reaction as endothermic or exothermic. B G Reaction coordinate Energy ... Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the ...
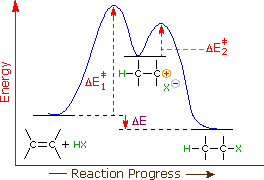
How does the energy level diagram show this reaction is exothermic? Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show: (a) the total energy content of the reactants compared to the total energy content of the products. (b) the energy difference between the reactants and the products, ΔH, heat of reaction Question #62891 | Socratic The figure above represents the reaction profile of a two step, exothermic reaction. The y-axis represents the potential energy of the reaction species, and the x-axis represents the progress of the reaction. The reaction is exothermic because the energies of the products are lower than those of the reactants. The reactants are represented by the horizontal line at the far left of the graph ... Solved Label the energy diagram for a two-step reaction. - Chegg Question: Label the energy diagram for a two-step reaction. enthalpy change transition state starting materials RX+H products rate-limiting transition state intermediates activation energy reaction coordinate This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (55 ratings) Question : Label the energy diagram for a two-step reaction. - Chegg Expert Answer. 100% (192 ratings) Labelle …. View the full answer. Transcribed image text: Label the energy diagram for a two-step reaction.
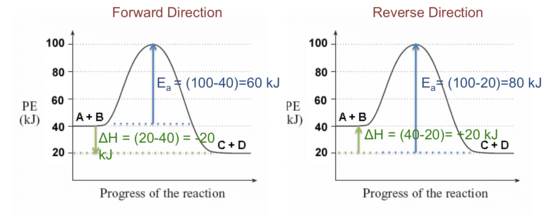
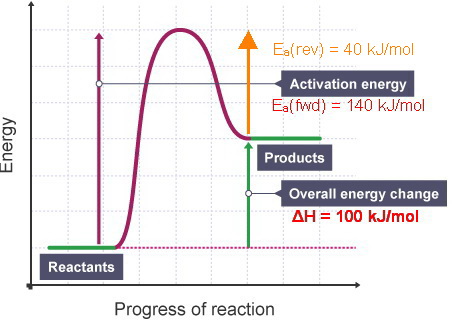
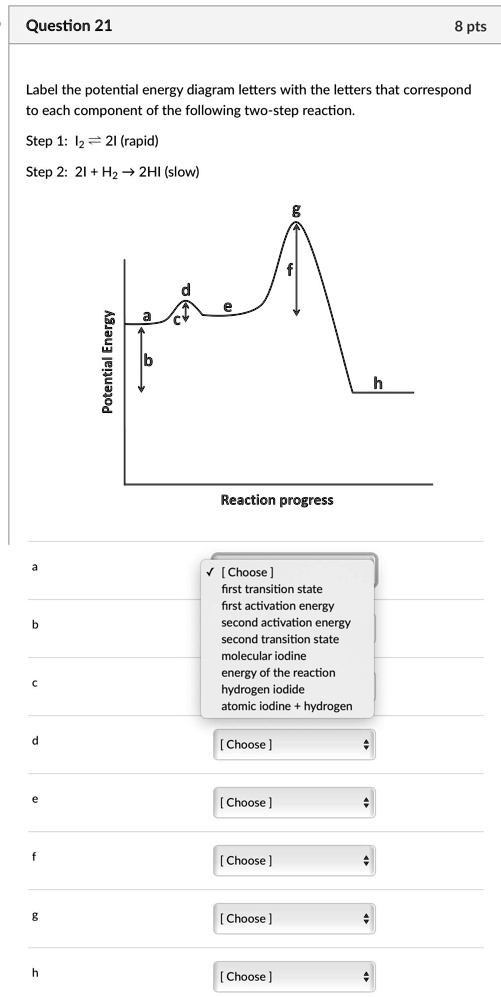
(a) Draw a reaction-energy diagram for the following reactio | Quizlet (a) Draw a reaction-energy diagram for the following reaction: \cdot \mathrm {CH}_ {3}+\mathrm {Cl}_ {2} \longrightarrow \mathrm {CH}_ {3} \mathrm {Cl}+\mathrm {Cl} ⋅CH3 +Cl2 CH3Cl+ Cl The activation energy is 4 kJ mol (1 kcal mol), and the overall \Delta H^ {\circ} ΔH ∘ for the reaction is -109 kJ/mol (-26 kcal/mol).
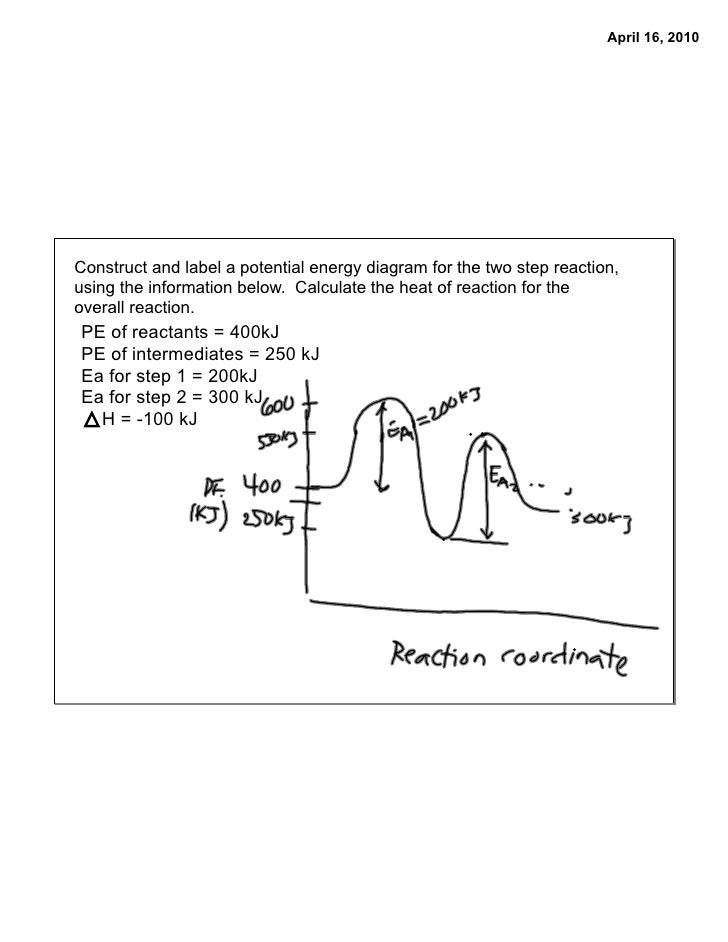
How to draw the potential energy diagram for this reaction? 2. Identify the sign of the enthalpy change, ΔH, along with its value. The decrease in chemical potential energy should be the same as the amount of thermal energy released. That is. Ereactants − Eproducts = 2219.9lkJ⋅ mol−1. However, since. ΔH = Eproducts −Ereactants , ΔH = −2219.9lkJ⋅ mol−1. Note that by convention, the ...
Answered: Label the components of an energy… | bartleby Q: To draw: An energy diagram for a two-step reaction in which both steps are exergonic and in which… A: The exergonic reactions are referred to as exothermic reactions in which, the reactant energy is…
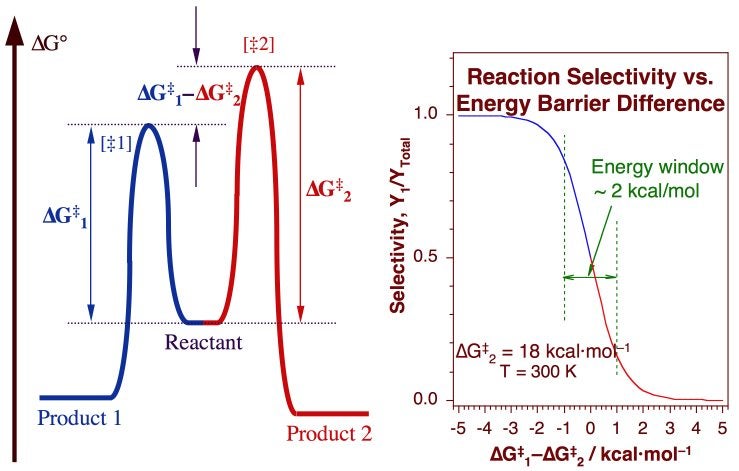
Sketch an energy diagram for a two-step reaction in which both steps ... Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram corresponding to reactant, product, intermediate, overall {eq}\Delta G^{\ddagger}{/eq}, and overall {eq}\Delta G^{\circ}{/eq}
Potential Energy Diagrams - Kentchemistry.com Answer-->. KNO 3 NH 4 Cl NH 4 NO 3 NaCl. 6/04. 21 A catalyst increases the rate of a chemical reaction by. (1) lowering the activation energy of the reaction (2) lowering the potential energy of the products. (3) raising the temperature of the reactants (4) raising the concentration of the reactants. Answer-->.
Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product.
SOLVED:Draw a reaction coordinate diagram for a two-step ... - Numerade here for the reaction. Coordinate diagram. Put two step verification in which the first step, Andrea Janek, the second step is extra, Janek. And the order reaction is and a sonic labels are re intense. Products, intermediates and chronic transition is start stairs.
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
Mechanisms and Potential Energy Diagrams | Chemistry for Non-Majors ... A potential energy diagram for a two-step reaction is shown and labeled. Practice View the section on two-step reactions at the site below and then do the self-test (both buttons are at the top of the slide). Don't worry about - just consider it an indication of activation energy as is in the diagram above.
Label the energy diagram for a two-step reaction. Draw a labeled reaction-energy diagram for a two-step reaction with an early first transition state and a late second transition state. State whether the transition states resemble the starting material, intermediate, or product. Repeat the exercise for a; Draw a stepwise mechanism for the reaction depicted below.


















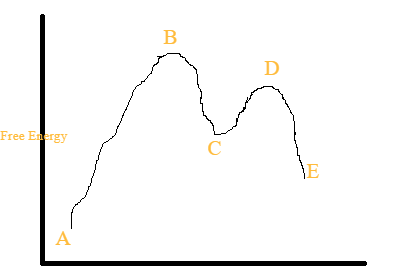

Post a Comment for "42 label the energy diagram for a two-step reaction."