40 open-label extension study
Health-Related Quality of Life Outcomes With Tofacitinib Treatment in ... AbstractBackground. Tofacitinib is an oral small molecule Janus kinase inhibitor for the treatment of ulcerative colitis. We report health-related quality of li Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1]
pubmed.ncbi.nlm.nih.gov › 34597534Long-term safety and efficacy of dupilumab in patients with ... Methods: TRAVERSE was an open-label extension study in 362 hospitals and clinical centres across 27 countries that assessed the safety and efficacy of dupilumab 300 mg every 2 weeks up to 96 weeks in adults and adolescents (aged 12-84 years) with moderate-to-severe or oral-corticosteroid-dependent severe asthma who had completed a previous ...

Open-label extension study
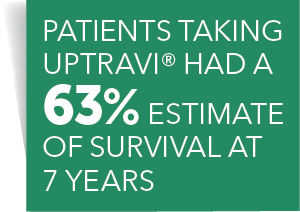
Spotlight on Open-Label Extension Studies The stated objective of most OLE studies is to obtain long-term safety and tolerability data. Open-label extension (OLE) studies are common, but they do not receive as much attention as traditional Phase I through Phase IV studies. Enrollment into an OLE study typically follows enrollment into a randomized, blinded, well-controlled main study. Open-Label Extension Study | UPTRAVI® (selexipag) HCP In long-term follow-up of patients who were treated with UPTRAVI® in the placebo-controlled study (N=574) and the open-label extension study (N=330, of 574), Kaplan-Meier estimates of survival at 1, 2, 5, and 7 years were 92%, 85%, 71%, and 63%, respectively. Prolia® (denosumab) Open-Label Extension Study Design | Prolia® The open-label extension study is not blinded, not controlled, and includes inherent self-selection bias. A total of 351 patients (7.7%) had adverse events that led to discontinuation of Prolia ® and 277 patients (6.1%) had adverse events that led to discontinuation of the study. 1,4
Open-label extension study. › quote › stockFulcrum Therapeutics to Present New Data from the Open Label ... Oct 13, 2022 · CAMBRIDGE - Fulcrum Therapeutics, Inc (Nasdaq: FULC), a clinical-stage biopharmaceutical company focused on improving the lives of patients with genetically defined rare diseases, announced today that new data from the open label extension (OLE) portion of the Phase 2 ReDUX4 study of losmapimod for the treatment of FSHD will be featured in a poster presentation at the World Muscle Society (WMS ... Amgen Announces Results From Two Open Label Extension Studies of ... Detailed study results will be shared with regulatory authorities and submitted for presentation at an upcoming medical congress later this year. Prolonged LDL-C reduction with Repatha is also being studied in the ongoing VESALIUS-CV (NCT03872401) outcomes trial. Repatha ® Cardiovascular Open-Label Extension (FOURIER-OLE) Study Design ... Open Label Expansion - Athira Clinical Trials Open Label Expansion - Athira Clinical Trials Participating in the Open Label Extension Upon completion of either ACT-AD or LIFT-AD study, interested participants are eligible to enroll in the Open Label Extension study of fosgonimenton. This study provides an opportunity for interested participants to receive the active drug during this study. Open label extension studies and patient selection biases Methods: The usual method of analysis of the open label extension study, which ignores any patients not continuing into the follow-on period of the study, is outlined. It is shown that ignoring patients who exit the trial is equivalent to assuming the outcome data are missing completely at random.
Open-label Extension Study of Efficacy, Safety and Tolerability of ... This study is an up to three-year open-label extension study to the core study CAIN457Q12301 (a two-year, phase III study that enrolled participants aged 18 to 75 years with active Lupus Nephritis). Open-label extension studies: do they provide meaningful ... - PubMed Consumers, institutions where these studies are conducted and research ethics committees need to be convinced of the motives, as well as the quality, of the open-label extension study and its execution before supporting such studies. Open-label extension studies do have a legitimate but limited place in the clinical development of new medicines. End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time. Safety and effectiveness of ulotaront (SEP-363856) in ... - Nature The aim of this 26-week open-label extension study was to evaluate the safety and effectiveness of ulotaront (25/50/75 mg/d) in patients who completed the initial 4-week study. Of the 193 4-week ...
Open-Label Extension Studies | SpringerLink - Acta Mechanica As the name implies, an open-label extension study is an 'appendage' to a randomised controlled clinical trial, usually of an unregistered medicine or intervention. Often the drug is being studied under an investigational new drug (IND) licence or equivalent legislation. The open-label extension study is identified formally as a study. Lower injection‐site reactions and long‐term safety, immunogenicity ... This open-label extension study evaluated the long-term safety, efficacy, and immunogenicity of YLB113 50 mg (once every 2 weeks) in patients with RA who were previously treated with etanercept RP and YLB113 for 52 weeks during a phase III study. In this study, the sustainability of long-term administration of YLB113 50 mg for up to 96 weeks ... Consent to open label extension studies: some ethical issues A frequent feature of pharmaceutical research is the open label extension study, in which patients participating in double blind placebo controlled trials of new medications are invited, on completion of the initial trial, to take the study drug for some further period. Patients are openly given the active substance at this stage, regardless of their assignment in the initial trial. Understanding Clinical Trial Terminology: What is an Open Label ... Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete one clinical trial may be eligible to continue in an open-label extension study where all participants are eligible to receive active treatment for an ...
› content › 378Viscosupplementation for knee osteoarthritis: systematic ... Jul 06, 2022 · A 26 week, double-blind, randomized, placebo-controlled study of the efficacy and safety of single intra-articular injection 1.2% sodium hyaluronate for treatment of painful osteoarthritis of the knee, with optional 26-week open-label safety extension).
NOPARK Open Label Extension Study - Full Text View - ClinicalTrials.gov The NOPARK open label extension study is an open label study, where subjects enrolled in the NOPARK study (ClinicalTrials.gov: NCT03816020), upon completion of the study, will be offered the NR study drug for continuous use, until the NOPARK study is completed and the primary end-point assessed (31/12/2025).
clinicaltrials.gov › ct2 › showA Study to Investigate the Efficacy and Safety of Two Doses ... May 16, 2018 · A Phase II, Open Label, Randomized, Two-Arm Study to Investigate the Efficacy and Safety of Two Doses of the Antibody Drug Conjugate GSK2857916 in Participants With Multiple Myeloma Who Had 3 or More Prior Lines of Treatment, Are Refractory to a Proteasome Inhibitor and an Immunomodulatory Agent and Have Failed an Anti-CD38 Antibody (DREAMM 2)
Cassava Sciences Announces Initiation of an Open-label Open-label Extension Study Design This multi-center, multi-national, open-label extension study is expected to generate long-term safety and tolerability data for (oral) simufilam 100 mg twice...
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials can be used to gather additional safety and efficacious data on drugs on the market to increase the confidence of clinicians, patients, and clinical bodies. They can play a key...
Understanding Clinical Trial Terminology: What is a Long-Term Extension ... An OLE study is a clinical trial that typically enrolls participants of a previous clinical trial and is designed to gather the long-term safety and tolerability data on a potential new medicine beyond the time period of the main study.
What is an open label extension study? • NCK Pharma Tags What is an open label extension study? An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered.
› article › releasesCassava Sciences Announces Initiation of an Open-label ... Oct 13, 2022 · Open-label Extension Study Design This multi-center, multi-national, open-label extension study is expected to generate long-term safety and tolerability data for (oral) simufilam 100 mg twice daily over 52 weeks. Each clinical investigational site must choose whether to participate in this open-label extension study. Patient enrollment for ...
Open label extension studies: research or marketing? - PMC Open label extension studies typically follow a double blind randomised placebo controlled trial of a new drug. At the end of the double blind phase, participants are invited to enrol in an extension study. The study will normally be longer than the randomised trial (two years is not uncommon but they often continue until the drug is licensed).
› news › homeKalVista Pharmaceuticals Announces Initiation of KONFIDENT-S ... Aug 23, 2022 · Initiation of this OLE study follows submission to the FDA of pivotal toxicology studies intended to support the eventual NDA filing. For more information on the open-label extension study, please ...
Cassava Sciences Announces Initiation of an Open-label Extension Study Open-label Extension Study Design This multi-center, multi-national, open-label extension study is expected to generate long-term safety and tolerability data for (oral) simufilam 100 mg twice...
Open-label Extension Study Evaluating Long-term Safety and Efficacy of ... Study design. FX2016-13 (Study 13) was an open-label, multicenter, 40-week extension study that was conducted at 70 sites in the United States. Subjects were eligible to enter this study upon successful completion of one of two pivotal, double-blind, vehicle-controlled, Phase 3 studies, FX2016-11 (Study 11) and FX2016-12 (Study 12).
Open Label Extension Study Definition | Law Insider Examples of Open Label Extension Study in a sentence. Rigel shall conduct such Open Label Extension Study in a good scientific manner, in compliance in all material respects with all Applicable Laws and all applicable portions of the Transition Plan.. AZ shall be solely responsible for the development of the Products in the Field in the Territory, and shall assume responsibility to fund the On ...
Cassava Sciences Announces Initiation of an Open-label Extension Study Open-label Extension Study Design This multi-center, multi-national, open-label extension study is expected to generate long-term safety and tolerability data for (oral) simufilam 100 mg twice daily over 52 weeks. Each clinical investigational site must choose whether to participate in this open-label extension study.
5-Year Open-Label Extension Data - Ocaliva® (obeticholic acid) for HCPs Open-label extension study design a In the 5 mg→10 mg titration group, 36 patients stayed at 5 mg and 33 were titrated to 10 mg after 6 months. 1 b Among patients who entered the open-label extension, 13 (7%) were intolerant and did not receive concomitant UDCA during double-blind or open-label treatment with OCALIVA. 3
argenx Presents Interim Results from ADAPT+ Open-Label Extension Study ... argenx se (euronext & nasdaq: argx), a global immunology company committed to improving the lives of people suffering from severe autoimmune diseases, today announced interim results from adapt+, an ongoing phase 3, open-label, three-year extension study evaluating long-term safety, tolerability and efficacy of vyvgart® (efgartigimod alfa-fcab) …
annualmeeting.acaai.orgACAAI Annual Scientific Meeting 2022 2022 Annual Scientific Meeting. Nov. 10-14, 2022 Kentucky International Convention Center Louisville, Kentucky Podium to Practice TM Advancing Allergy & Immunology Care
Prolia® (denosumab) Open-Label Extension Study Design | Prolia® The open-label extension study is not blinded, not controlled, and includes inherent self-selection bias. A total of 351 patients (7.7%) had adverse events that led to discontinuation of Prolia ® and 277 patients (6.1%) had adverse events that led to discontinuation of the study. 1,4
Open-Label Extension Study | UPTRAVI® (selexipag) HCP In long-term follow-up of patients who were treated with UPTRAVI® in the placebo-controlled study (N=574) and the open-label extension study (N=330, of 574), Kaplan-Meier estimates of survival at 1, 2, 5, and 7 years were 92%, 85%, 71%, and 63%, respectively.
Spotlight on Open-Label Extension Studies The stated objective of most OLE studies is to obtain long-term safety and tolerability data. Open-label extension (OLE) studies are common, but they do not receive as much attention as traditional Phase I through Phase IV studies. Enrollment into an OLE study typically follows enrollment into a randomized, blinded, well-controlled main study.

























Post a Comment for "40 open-label extension study"