39 ch2br2 hybridization
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. CH2Br2 b. SO2 c. NF3 d. BF3 H2S Lewis Structure, Molecular Geometry, Hybridization and Polarity To understand the hybridization of H2S, it is vital to know two things first: The number of bonds in the compound and its type It is essential to know the type of bonding in the compound to know its hybridization. In the H2S molecule, two Hydrogen atoms form a bond with the central Sulfur atom. Two single bonds are formed in the molecule.
How to Predict the Type of Hybridization in a Molecule or Ion Step 1: Add the number of valence electrons of all the atoms present in the given molecule/ion. Step 2: In case of a cation, subtract the number of electrons equal to the charge on the cation and in case of an anion, add number of electrons equal to the charge on the anion. Step 3: (i) If the result obtained in step 2 is less than 8, divide it ...
Ch2br2 hybridization
Solved What is the hybridization of C in CH2I2? | Chegg.com What is the hybridization of C in CH2I2? Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. In CH2I , the C atom forms 4 sigle bonds (1 with each of the two H atoms an …. View the full answer. Finding the hybridization of atoms in organic molecules (worked ... All right, let's move over to this carbon, right here, so this carbon has a triple-bond on the right side of it, and so the fast way of doing this, is if it has a triple-bond, it must be SP hybridized here, so SP hybridized, and therefore, the geometry would be linear, with a bond angle of 180 degrees. Dibromomethane | CH2Br2 - PubChem Dibromomethane is a member of the class of bromomethanes that is methane substituted by two bromo groups. It is produced by marine algae. It has a role as a marine metabolite and an algal metabolite. It is a member of bromomethanes and a bromohydrocarbon.
Ch2br2 hybridization. Hybridization - sp, sp2, sp3, sp3d, sp3d2 Hybridized Orbitals ... - BYJUS Hybridization in Chemistry is defined as the concept of mixing two atomic orbitals to give rise to a new type of hybridized orbitals. This intermixing usually results in the formation of hybrid orbitals having entirely different energies, shapes, etc. The atomic orbitals of the same energy level mainly take part in hybridization. Ch2br2 hybridization 4. Identify the hybridization of the B atom in BF3. label all bonds in ch2br2 extraordinary label all bonds in ch2br2 with solved write a hybridization and scheme for each molec of label all bonds in ch2br2 {Label Gallery} Get some ideas to make labels for bottles, jars, packages, products, boxes or classroom activities for free. Identify the orbitals that overlap to form the C-Br bonds in CH2Br2 a ... Overlap between various atomic orbitals produces hybrid orbitals. These hybrid orbitals have the same shape but dissimilar orientations. Answer and Explanation: 1 Become a Study.com member to... What is the molecular geometrical structure of C2H2Br2 and its ... Lewis structure for c2h2br2? The Lewis structure for 1,2 - dibromoethylene starts with a pair of doubly bonded C atoms in the center. Each carbon then has two single bonds, one attached to an H...
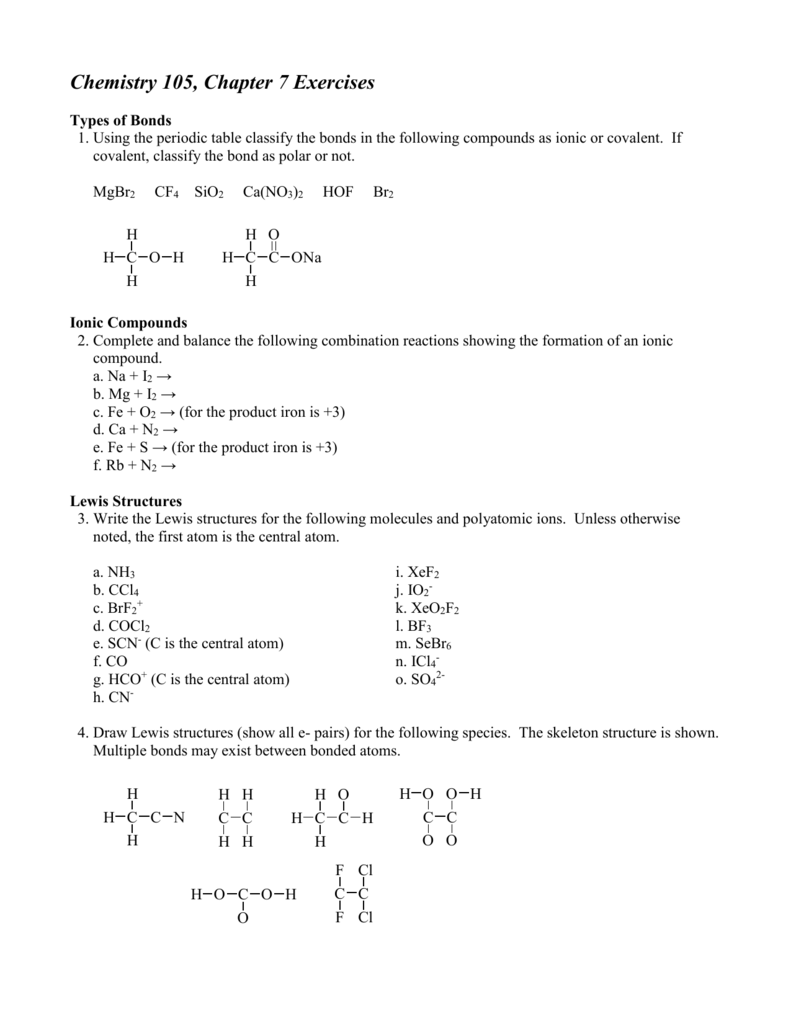
What hybridization is expected on the central atom of each of the ... V=Valence electrons of central atom. M- Number of monovalent atoms linked to central atom. C= Charge of cation. A= Charge of anion. Consider the hybridization of the options:- A) BeH 2 - H= 21 [2+2]=2. ⇒sp hybridized state. B) CH 2 Br 2 H= 21 [4+4]=4. ⇒sp 3 hybridized state. C) PF 6− H= 21 [5+6+1]=6. ⇒sp 3d 2 hybridized state. D) BF 3 H= 21 H2S Molecular geometry, SH2 Lewis structure, Bond angle, Shape 2 lone pairs on the sulfur central atom. Therefore, the generic formula of hydrogen sulfide is AX2N2 for finding the molecular or electron geometry of H2S. So, AX2N2 gives the molecular geometry of H2S is bent and the electron geometry is tetrahedral according to VSEPR Shape Chart. The bond angle of H2S is 92.1°. CH2Cl2 lewis structure, molecular geometry, polarity | Dichloromethane Hybridization of Dichloromethane When two or molecules participate in the bond formation, their orbitals overlap due to the sharing of electrons. These overlapped orbitals are called hybrid orbitals. The bonds formed in Dichloromethane are covalent bonds. Central Carbon is hybridized as the molecule forms all the four bonds in the compound. Solved 1.Identify the hybridization of the C atom in | Chegg.com See the answer 1.Identify the hybridization of the C atom in CH2Br2. 2. Identify the hybridization of the S atom in SO2. 3. Identify the hybridization of the N atom in NF3. 4. Identify the hybridization of the B atom in BF3. Expert Answer 99% (105 ratings) Previous question Next question
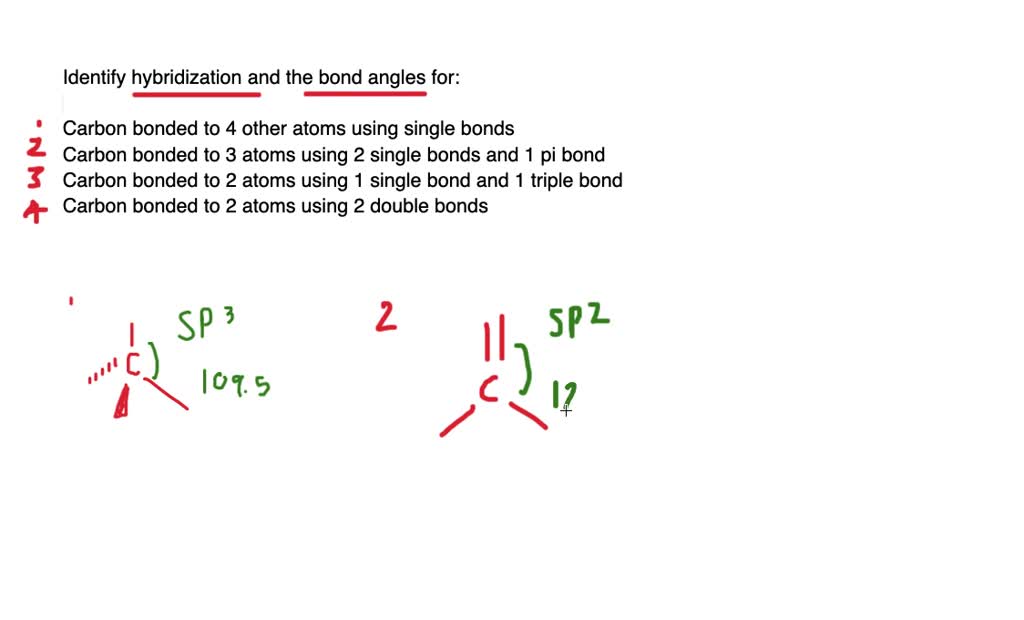
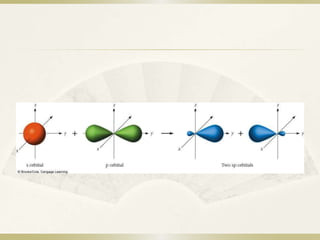
vsepr theory - How do I figure out the hybridization of a particular ... Hybridization was invented to make quantum mechanical bonding theories work better with known empirical geometries. If you know one, then you always know the other. Linear - s p - the hybridization of one s and one p orbital produce two hybrid orbitals oriented 180 ∘ apart. sp3, sp2, and sp Hybridization in Organic Chemistry with Practice ... One hydrogen bonds to each carbon atom by overlapping its s orbital with the other sp orbital. The two p orbitals of each carbon overlap to make two π bonds. The key parameters about the sp hybridization and triple bond: * All the atoms have linear geometry. * The angle between atoms is 180 o. Answered: Write a hybridization and bonding… | bartleby CH2Br2 b. SO2 c. NF3 d. BF3. Question. Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. CH2Br2 b. ... What is the hybridization of the oxygen atom in the -CH2OH group? CH2Br2 Lewis Structure, Geometry, Hybridization, and Polarity In CH2Br2, C is the central atom. We are focusing on the hybridization of the central atom only. In the ground state, C has 2 unpaired electrons. It can only form two bonds. Promotion of electrons takes place, and all 4 valence electrons become unpaired. These 4 electrons are present in different orbitals.
C2H2Br2 Lewis Structure: How to Draw the Lewis Structure for C2H2Br2. A step-by-step explanation of how to draw the C2H2Br2 Lewis Dot Structure (1,2-Dibromoethylene).For the C2H2Br2 structure use the periodic table to find the ...
Use valence bond theory to write the hybridization and ... - Socratic Warning! Long Answer. Here's what I get. > Step 1. Draw the Lewis structure (a) Start with a skeleton structure. The two "C" atoms (least electronegative) will be the central atoms, with the "N" attached to one of the carbons. (b) Attach the hydrogen atoms. The question gives you a clue where they go. The formula "NCCH"_3 tells you that the three "H" atoms are attached to the terminal carbon atom.
2022 UPDATED!!! dentify the hybridization of the C atom in CH2Br2 ... The central atom is generally considered to be the electrostatic central atom. Calculate hybridization as follows: 1. If the value of X is 2 then it means that two hybrid orbitals are to be formed and the hybridization of the sp. 2. If X is a value of 3 then it means that three hybrid orbitals are to be formed and that they are hybridized . 3.
Identify the hybridization of the c atom in ch2br2. - Brainly.com The hybridization of the C atom in CH₂Br₂ is sp3 When bonding, the orbitals "s" and "p" from C atoms interact to form hybridized orbitals. If the C atom has 4 sigma bonds, as is the case in CH₂Br₂, there are 4 hybridized orbitals required, so 1 "s" orbital and 3 "p" orbitals hybridize to form a Unlock 15 answers now and every day
What type of hybridIzation is c2br2? - Answers Best Answer. Copy. There wont be a stable compound with the formula C2Br2. If there is then it will be sp hybridization of carbon. If the question is for CH2Br2, then carbon will be sp3 hybridized ...
What is the electron geometry, molecular geometry, and hybridization of ... The electron geometry and molecular geometry are different. The arrangement of electron groups around the central atom whether bonding or non-bonding is known as the electron geometry. Molecular ...
Krf2 Hybridization Of Central Atom - backstory-nextchapter KrF2 Kr NH2Cl N CH2Br2 C SCN-1 C Could someone please show me how this is done. 3 Chemical and Physical Properties Expand this section. ... Krf2 hybridization of central atom. Is KrF2 linear. The stabilities of the dihalides follow the order KrF2 XeF2 RnF2 and XeF2 XeCl2 XeBr2. KRF2 XEOF4 03 BI3 Give the hybridization of the central atom ...
How is the hybridization of SO3 2-determined? - Quora Answer (1 of 4): 1. Draw the Lewis structure. 2. Count the domains around the S where you find electrons. A domain is a region in space where you find electrons. I see 4 here: a lone pair, two single bonds and a double bond. 3. You need the same number of hybrid orbitals as you have domains. In...
Dibromomethane | CH2Br2 - PubChem Dibromomethane is a member of the class of bromomethanes that is methane substituted by two bromo groups. It is produced by marine algae. It has a role as a marine metabolite and an algal metabolite. It is a member of bromomethanes and a bromohydrocarbon.
Finding the hybridization of atoms in organic molecules (worked ... All right, let's move over to this carbon, right here, so this carbon has a triple-bond on the right side of it, and so the fast way of doing this, is if it has a triple-bond, it must be SP hybridized here, so SP hybridized, and therefore, the geometry would be linear, with a bond angle of 180 degrees.
Solved What is the hybridization of C in CH2I2? | Chegg.com What is the hybridization of C in CH2I2? Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. In CH2I , the C atom forms 4 sigle bonds (1 with each of the two H atoms an …. View the full answer.

























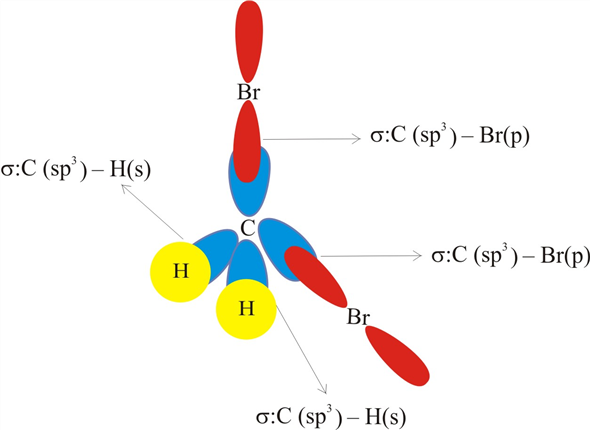




Post a Comment for "39 ch2br2 hybridization"