44 what term is used to label the energy levels of electrons
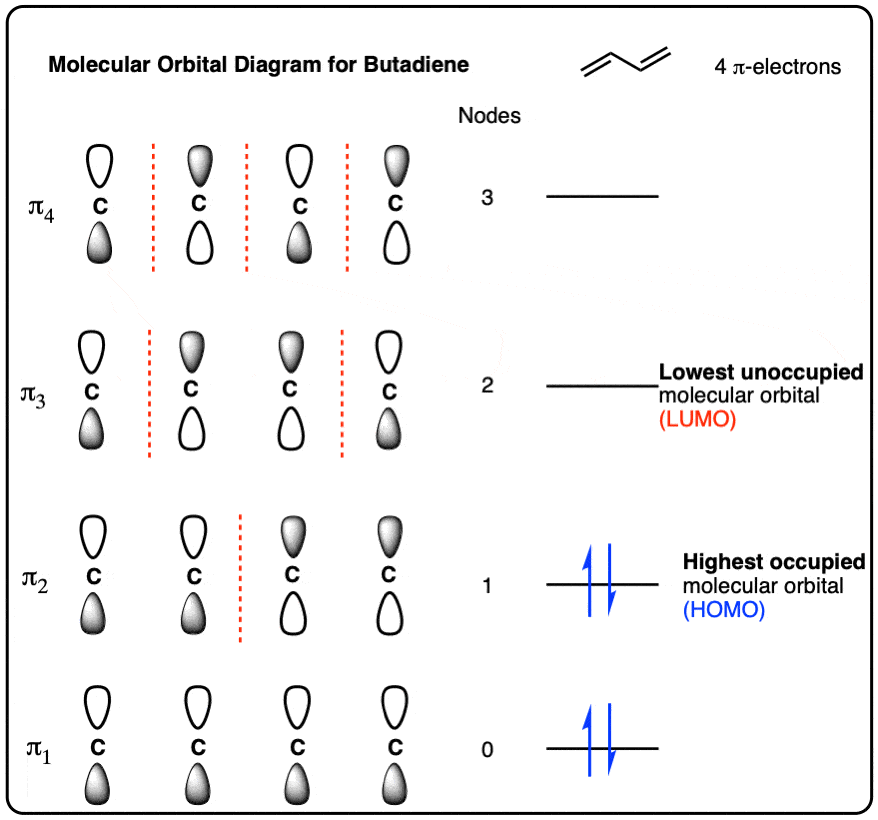
Term Symbols for Atomic Energy Levels Term Symbols for Atomic Energy Levels Term Symbols The heirarchy of labels for the electrons of multi-electron atoms is configuration, term, level, and state. The term uses the multiplicity 2S + 1, total orbital angular momentum L, and total angular momentum J. Bohr model energy levels (derivation using physics) The thing is that here we use the formula for electric potential energy, i.e. the energy associated with charges in a defined system. The Formula for electric potenial = (q) (phi) (r) = (KqQ)/r. We use (KqQ)/r^2 when we calculate force between two charges separated by distance r. This is also known as ESF.
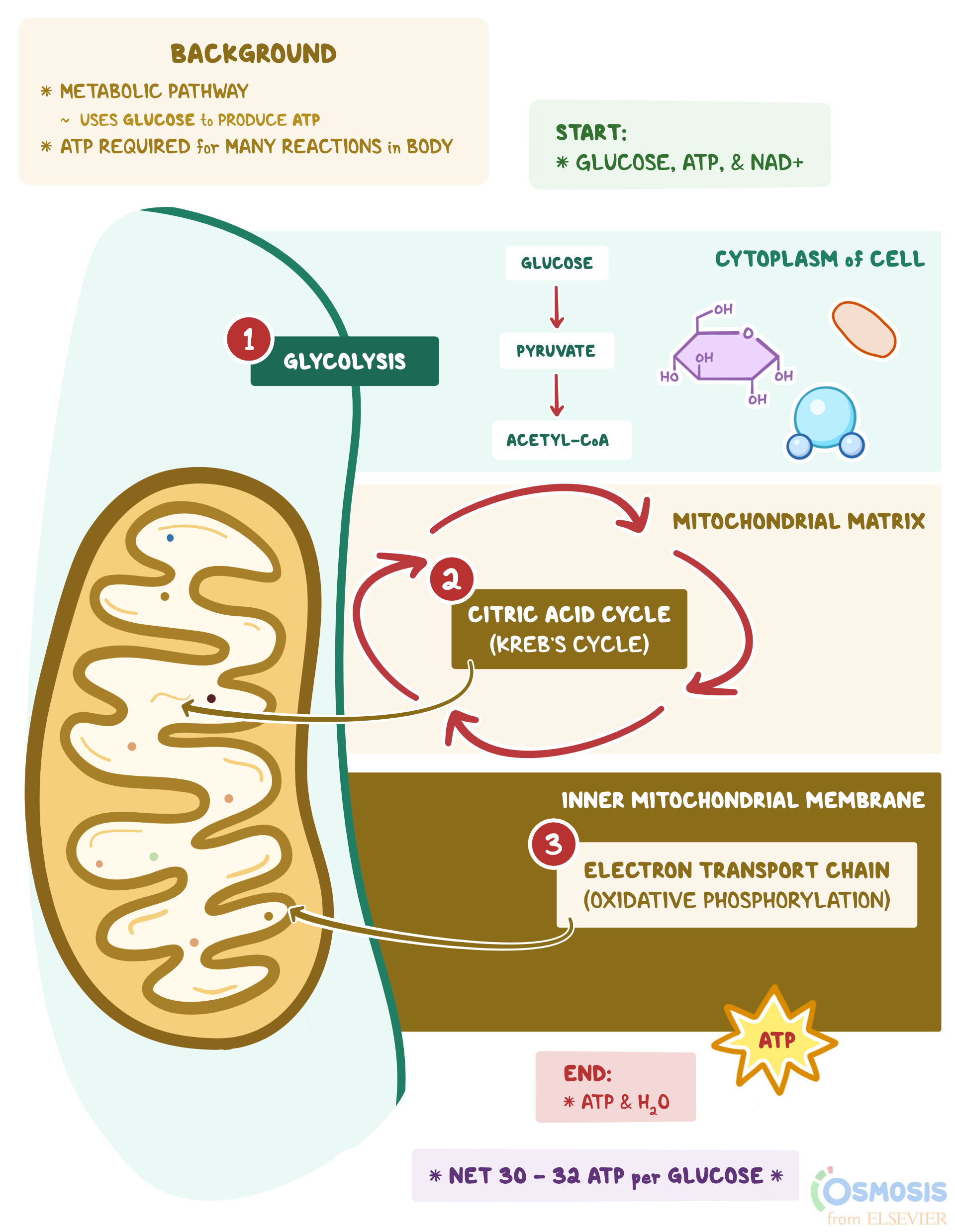
8.3: Electron Configurations- How Electrons Occupy Orbitals Aug 14, 2020 · The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 8.3. 3 or 8.3. 4 ). Thus, the electron configuration and orbital diagram of lithium are:
What term is used to label the energy levels of electrons
Atomic Energy Levels (video) | Khan Academy The electron can absorb photons that will make it's charge positive, but it will no longer be bound the the atom, and won't be a part of it. For example at -10ev, it can absorb, 4eV (will move to -6eV), 6eV (will move to -4eV), 7eV (will move to -3eV), and anything above 7eV (will leave the atom) 2 comments ( 12 votes) Upvote Downvote Flag more What term is used to label the energy levels of electrons? Nov 13, 2019 · Answer : The term used to label the energy levels of electrons is, Principle Quantum Numbers (n). Explanation : There are 4 quantum number. Principle Quantum Numbers : It describes the size of the orbital or energy levels of electrons. It is represented by n. n = 1,2,3,4.... Azimuthal Quantum Number : It describes the shape of the orbital. Chapter 5 test Section 5.1 Flashcards | Quizlet Nov 4, 2014 · The electrons in an atom can exist between energy levels. Energy levels What are the fixed energies of electrons called? Move an electron from its present energy level to a higher one A quantum of energy is the amount of energy required to? Closer In general, the higher the electron is on the energy ladder, the ______ it is from the nucleus
What term is used to label the energy levels of electrons. Chapter 5 test Section 5.1 Flashcards | Quizlet Nov 4, 2014 · The electrons in an atom can exist between energy levels. Energy levels What are the fixed energies of electrons called? Move an electron from its present energy level to a higher one A quantum of energy is the amount of energy required to? Closer In general, the higher the electron is on the energy ladder, the ______ it is from the nucleus What term is used to label the energy levels of electrons? Nov 13, 2019 · Answer : The term used to label the energy levels of electrons is, Principle Quantum Numbers (n). Explanation : There are 4 quantum number. Principle Quantum Numbers : It describes the size of the orbital or energy levels of electrons. It is represented by n. n = 1,2,3,4.... Azimuthal Quantum Number : It describes the shape of the orbital. Atomic Energy Levels (video) | Khan Academy The electron can absorb photons that will make it's charge positive, but it will no longer be bound the the atom, and won't be a part of it. For example at -10ev, it can absorb, 4eV (will move to -6eV), 6eV (will move to -4eV), 7eV (will move to -3eV), and anything above 7eV (will leave the atom) 2 comments ( 12 votes) Upvote Downvote Flag more
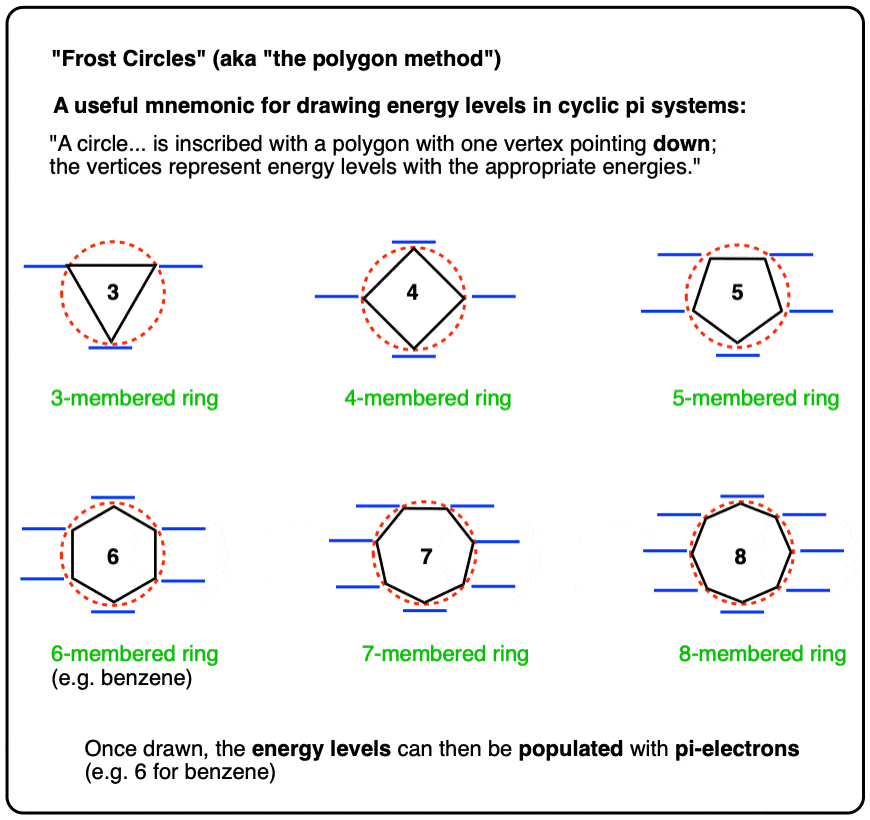

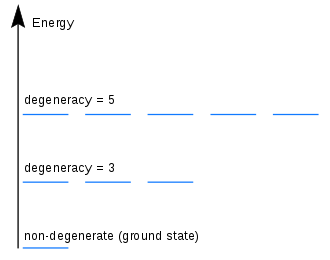






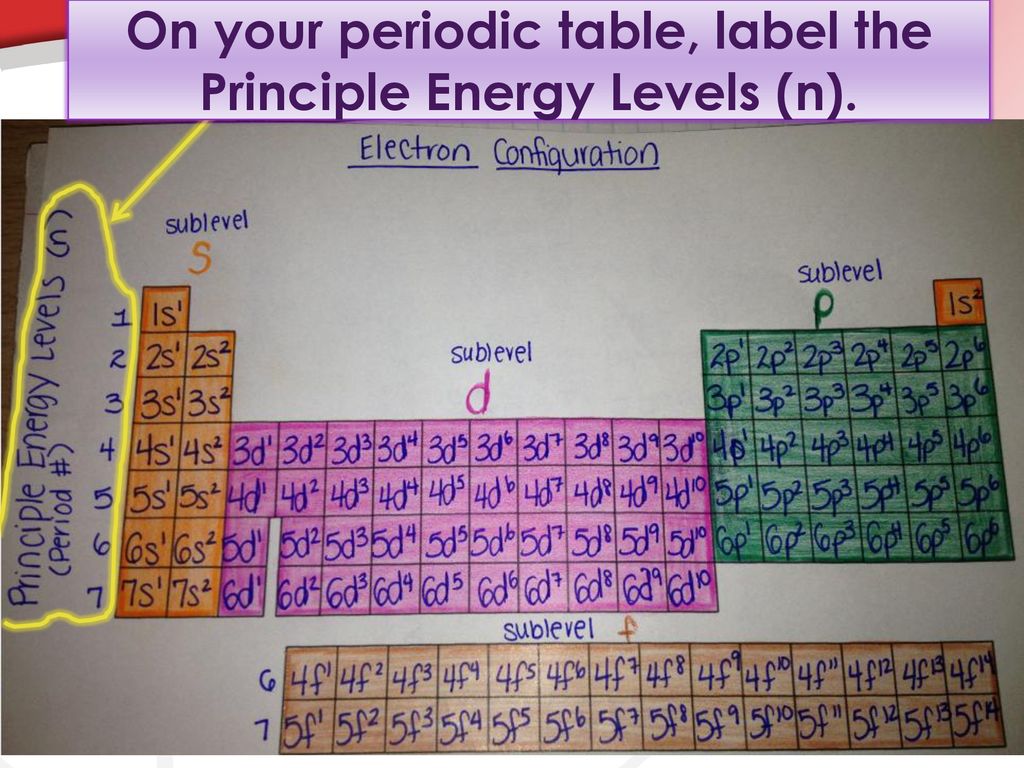

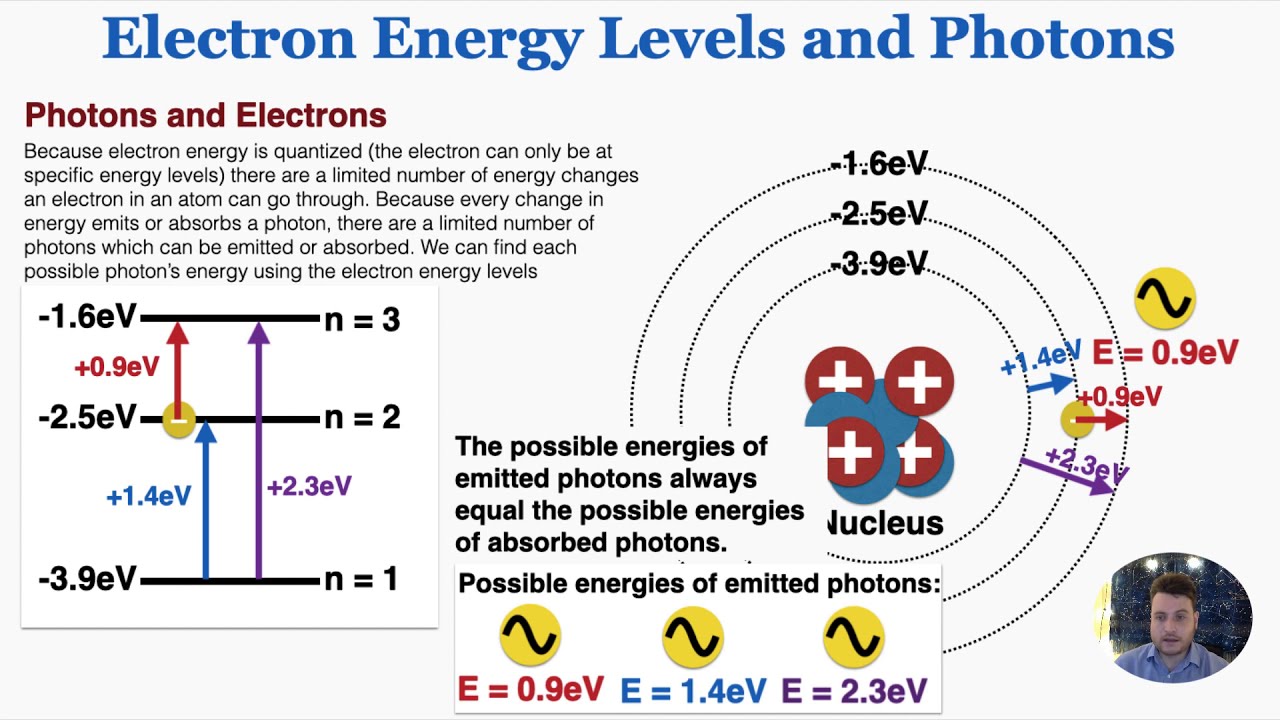
























Post a Comment for "44 what term is used to label the energy levels of electrons"